
CHILDREN
DESERVE
POWERFUL
PROTECTION
AS THEIR CAREGIVER,
YOU CAN PROVIDE IT
WITH IDELVION
With IDELVION, Factor IX stays in their body longer
With the longest single-dose half-life of any Factor IX therapy at 90-93 hours,*
IDELVION keeps Factor IX levels high enough to extend protection.
In the IDELVION pediatric pivotal trial, children had a median of 0 spontaneous bleeds per year when dosed every 7 days.

*Based on a single dose of IDELVION at 50 IU/kg in adults (< 12 years).
† Steady-state is a consistent and uniform amount of factor in the body from continued use.
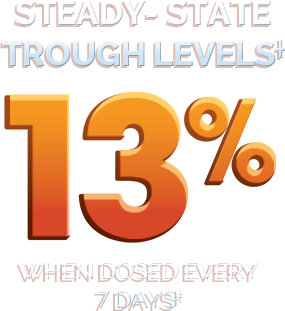
‡Only time points with ≥ 3 observations are plotted. Steady-state mean trough is calculated from all
observations at any time points. The average dose for patients receiving prophylaxix for every 7 days was 47 IU/kg.
Milinda spends less time driving to the hospital and more time with her family.
See the impact IDELVION has had on Milinda’s life
Consider extended dosing when it's time
High factor levels offer powerful bleed protection at the very start. After turning 12, patients may be able to work with their doctor to extend dosing and infuse less—only IDELVION is FDA approved for 14-day dosing in adolescents and adults.


Those 12 and older have the option to extend dosing to 14 days once they are well-controlled on 7-day prophylaxis.
*Once well‑controlled (1 month without spontaneous bleeding or requiring dose adjustments on a weekly dose of ≤40 IU/kg), people 12 years and older can be transitioned to 14-day dosing.
Talk to other people living with hemophilia B
IDELVION Connect can help you receive copay support, a free trial, and other resources

